At WiseGEEK, we're committed to delivering accurate, trustworthy information. Our expert-authored content is rigorously fact-checked and sourced from credible authorities. Discover how we uphold the highest standards in providing you with reliable knowledge.
What is the Nernst Equation?
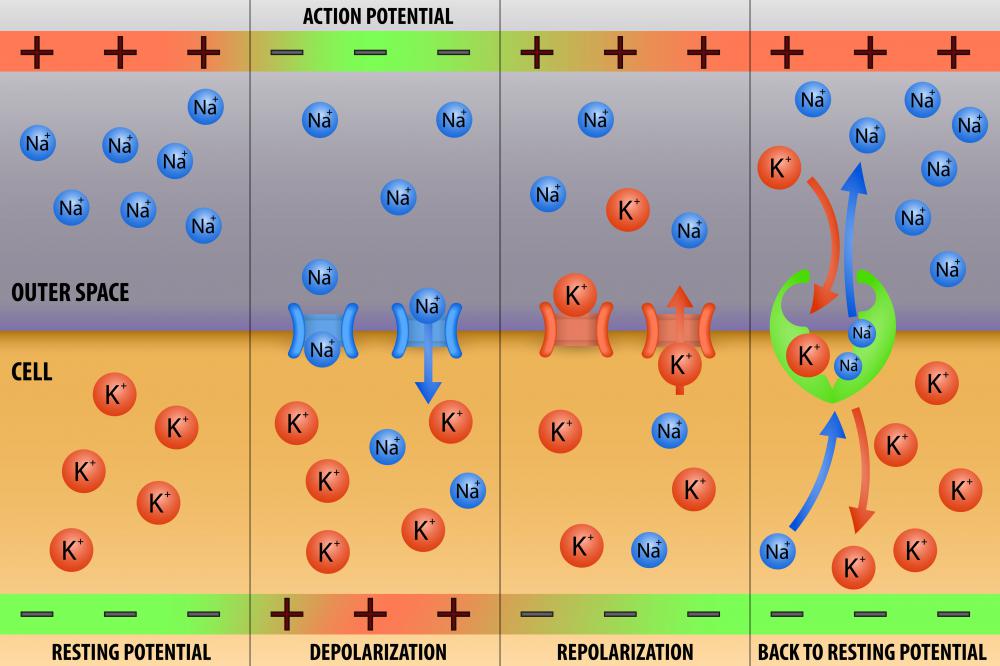
The Nernst equation determines the resting potential of cell membranes in the body as a factor of the concentration of ions inside and outside of the cell. Cells are the basic unit of the body, and the environment inside the cell is separated from the outside by a cellular membrane. The intracellular environment contains a concentration of ions that is different from that of the extracellular environment, so an electric charge develops, and it is referred to as the resting potential. The ions that are most influential in determining the resting potential are those to which the cell membrane is most permeable: sodium and potassium. There is a higher concentration of potassium inside the cell than outside the cell, and the opposite is true for the sodium ion.
For many of the cells in the body, the resting potential stays constant for the duration of cell life. For excitable cells such as those of the nerves and muscles, however, the resting potential simply refers to the membrane potential when the cell is not being excited. An excitable cell is one that generates an electrical impulse that causes the cell to contract, in the case of a muscle cell, or fire a signal, in the case of a nerve cell.

Excitation results in the changing of the membrane's permeability to ions, mainly potassium and sodium. This allows for the flow of ions from the area of higher concentration to the area of lower concentration, and this flow causes an electrical current that will change the charge across the membrane. Therefore, the Nernst equation is not applicable in this case, because the Nernst equation takes into account only ion concentration when there is no permeability across the cell membrane.
The Nernst equation factors in constants such as the Faraday constant, the universal gas constant, the absolute temperature of the body and the valence of the considered ions. Potassium is the most commonly considered ion in the equation. It is the ion of greatest permeability, so it flows across the membrane most.
The Nernst equation has been criticized because it assumes that there is no net flux of ions across the cell membrane. Realistically, there is never no net flux of ions, because ions escape due to leakage or are actively pumped by the cell across the membrane. In many cases, the more universal Goldman equation is preferred when predicting membrane potential. The Goldman equation takes into account the membrane's permeability to ions for a more accurate assessment of membrane potential, and it can be used for excitable and non-excitable cells.
AS FEATURED ON:
AS FEATURED ON:










Discuss this Article
Post your comments