At WiseGEEK, we're committed to delivering accurate, trustworthy information. Our expert-authored content is rigorously fact-checked and sourced from credible authorities. Discover how we uphold the highest standards in providing you with reliable knowledge.
What is Calreticulin?
Calreticulin is a protein found in all cells that have a membrane-bound nucleus, such as human and animal cells. The protein has multiple functions, including a role in maintaining calcium levels, helping to fold other proteins into the correct structure, cell signaling and cell death. Calreticulin can perform so many functions because the protein has three distinct areas suited to different uses.
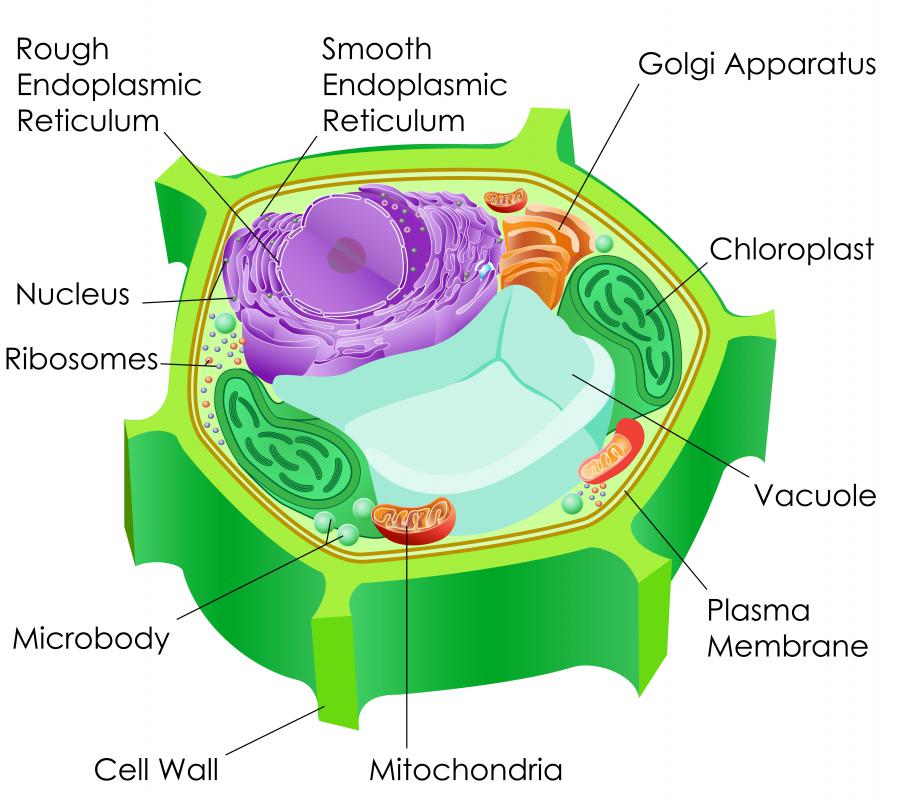
Calreticulin was discovered in 1974. The protein was found in the part of the cell called the endoplasmic reticulum, where proteins are assembled, modified and folded. It was initially understood to be a molecule that bound calcium and was involved in calcium storage, but more functions of the protein were identified in the 1990s.

The protein has three major parts, and each of these parts is used to perform different cell functions. The N-domain is at one end of the protein and is used in folding other proteins. The middle section is called the P-domain and is used in calcium binding and also for protein folding. The other end of the calreticulin protein, opposite the N-domain, is the C-domain, which is used for calcium binding and as a cellular signal.

The N-domain of calreticulin interacts with other proteins to help fold them correctly, but the domain also has other uses. It also inhibits the proliferation of endothelial skin cells and suppresses the growth of new blood vessels. The N-domain also binds with certain cell receptors and enzymes.
The middle part of the calreticulin protein, the P-domain, has an amino acid sequence that binds strongly to calcium. It also has a sequence that is attracted to sugar residues of proteins and is used in protein folding. The P-domain also interacts with immune system molecules involved in cell breakdown.
The C-domain has amino acid sequences that bind calcium to use in calcium regulation. The C-domain also has a specific amino acid sequence that acts as a signal to other molecules that the calreticulin belongs in the endoplasmic reticulum. This section of the protein also interacts with immune molecules, playing a role in blood clotting.
Calreticulin also appears to play a role outside the endoplasmic reticulum section of the cell. It is found in the nucleus, the body of the cell, outside the cell and on the cell surface. The protein has a part to play in wound healing, gene expression, cell adhesion, cell death and removal of cancer cells. These are in addition to its major roles as a protein folder and a calcium carrier.
AS FEATURED ON:
AS FEATURED ON:











Discuss this Article
Post your comments