At InfoBloom, we're committed to delivering accurate, trustworthy information. Our expert-authored content is rigorously fact-checked and sourced from credible authorities. Discover how we uphold the highest standards in providing you with reliable knowledge.
What Is the Difference between Fluorine and Fluoride?
The difference between fluorine and fluoride is fairly simple. Fluorine is an element and fluoride is the ion of element fluorine. Fluorine is one of the lightest elements among halogens. It is the most electronegative element with a value of 4 on the Pauling scale. Electronegative means the tendency to attract a bonding pair of electrons. Fluoride, on the other hand, is the negative ion of fluorine. Compounds containing the fluoride ion are referred to as fluorides. An example of a fluoride is sodium fluoride (Na+F-).
Fluorine was discovered as an element in other compounds in the early 1500s by the German physician Georgius Agricola. The element was isolated in its pure form by Henri Moissan in late 1800s. Fluorine can bond with almost any element due to its electronegative and oxidizing properties.

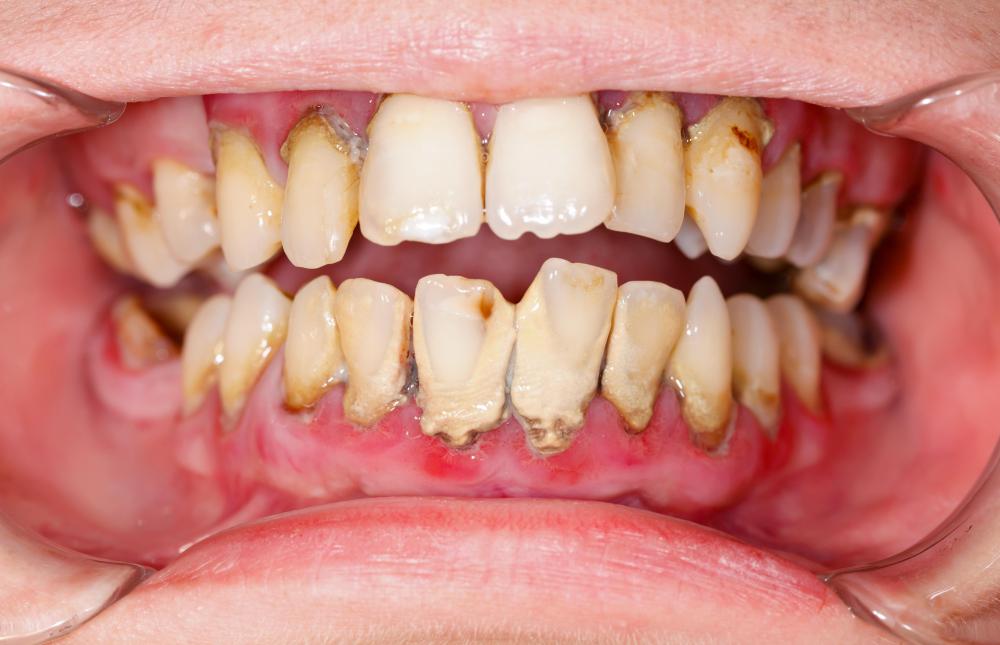
When hearing the term fluoride, most of us will generally think of toothpaste. Fuoride is found in nature but the fluoride added to drinking water and toothpaste is synthesized in the lab. In the human body, fluoride reacts with a form of calcium phosphate called hydroxyapatite in the bones and teeth to form fluoroapatite, which also occurs as a mineral. In the right amount, this appears to have a strengthening effect and provides significant protection against tooth decay and dental cavities. Despite its presence in the human body and its beneficial effects at the right levels, fluorine is not considered an essential element as humans appear able to live without it, and too much fluoride can be harmful.

There is some controversy surrounding the use of fluoride in products such as drinking water and toothpaste with the opponents emphasizing the potential harmful effects of excessive fluoride. Opponents of fluoridation of water supplies have argued that it is a form of enforced medication, while those in favor argue that it simply brings fluoride levels up to normal for areas that are deficient in this element.

Although elemental fluorine is highly toxic due to its reactivity, fluorides are generally less so. Nevertheless, ingestion of soluble metal fluorides in other than very small amounts can have serious toxic effects and for this reason, toothpaste and mouthwash should not be swallowed. The acute effects of fluoride ingestion include damage to the brain and kidneys and effects on the heart. The lethal dose for sodium fluoride is estimated at 0.175 – 0.353 ounces (5-10 grams), an amount very unlikely to be absorbed through contact with generally available products containing fluoride. The effects of chronic overexposure to fluoride include mottling of teeth, brittle bones, anemia and stiff joints.

Fluorine and fluoride are widely used in industry. One very useful fluorine-containing product is polytetrafluoroethylene (PTFE), a fluorinated plastic sold under a well-known trade name and used domestically on cooking utensils because of its heat resistance and non-stick properties. In addition, PTFE is used industrially for the storage of reactive substances, due to its chemical inertness. Fluorine and fluoride are also used in the production of pesticides, such as sulfuryl fluoride, and in an intermediate step in the enrichment of uranium for nuclear reactors.
AS FEATURED ON:
AS FEATURED ON:












Discussion Comments
Thanks for sharing this information. I have always been confused on the difference between fluorine and fluoride. I will share this post with my readers, they visit my site to browse fluorine slogans, so this info is much appreciated.
I am the anonymous poster no. 5. I would still like to know if having fluorine as a cleansing agent is just as bad or the same as adding fluoride to the water? I am not a chemist and chemical compounds don't answer my question. Thanks!
I can't seem to pin down the difference in fluorine and fluoride as far as drinking water is concerned. The city I just moved to uses fluorine as a cleansing agent. Some citizens are under the illusion they do not have fluoride in their drinking water, but fluorine is used. This can't be good can it?
Fluorine doesn't always take an electron. Fluorine can covalently bond to carbon. Fluoride is ionic fluorine.
@Feryll - Nobody is being made to drink water with fluoride. If you don't want the water being pumped by your water company then get your water from somewhere else. I know plenty of people who buy bottled water-- not that I think it is better than tap water. Most people in my community have wells, so they have more of a say in what they are drinking.
However, I think fluoride in water has been great for many poor communities. If you take a look at dental health since fluoride was added to water in poor communities, I think you will see a big improvement.
This is not something that I have given much consideration, but I do think individuals should have the right to say whether or not they want fluoride added to their drinking water. After all, fluoride doesn't improve the quality or the safety of the water we drink, so why do the powers that be get to say that more than 70 percent of the U.S. population has to drink fluoridated water?
I grew up drinking water straight from the ground via an old well that had been used for generations in my family. My grandparents used the well by drawing the water up with a bucket. Then they would carry the water into the house. By the time I came along pipes had been connected to the well and there was running water in the house.
As a kid, when I would visit my cousins who had city water I thought the taste of their water was terrible. It was a while before I asked my father about the taste, and he explained to me that fluoride was added to the city water for health reasons. I did wonder if my cousins were going to have healthier teeth than I did, but I certainly didn't want to trade water with them.
To this day, I don't like the taste of water with fluoride in it, and my teeth are fine by the way.
Post your comments